STAGE OF DEVELOPMENT
Researchers have performed in vivo tests in mice using BMNPs as drug-delivery and hyperthermia agents for cancer treatment. The assays following this therapy combination have shown better results than non-targeted chemotherapy, reducing tumor size more efficiently than the soluble drug and without side effects.
The technology has been tested in vitro for breast, liver and colon cancer and for bacterial infections. It has also tested successful for other applications, like enzyme fixation and recycling for biotechnological processes, and bacterial pathogens biosensors.
PATENT: BIOMIMETIC MAGNETIC NANOPARTICLES COMPRISING MAMC
- Number: ES201831064
- Priority date: 02 November 2018
- PCT application: PCT/ES2097070747
- Drafted by Hoffman-Eitle
The patent application for the invention as well as its production method was granted in Spain the 13th of January 2021, and has already been extended to the EU and USA.
This patent discloses compositions comprising biomimetic superparamagnetic nanoparticles comprising magnetite (BMNPs). The magnetic nanoparticles may be used as nanocarriers of medicaments, in particular, as nanocarriers of medicaments for the treatment of diseases with an associated marker that can be targeted, as, for example, cancer. Also, it includes the possibility to apply them in combination with other alternative treatment, the hyperthermia.
Also discloses a method to produce the composition, magnetoliposome formulation comprising such compositions, and their uses as medicament, contrast agent, nucleic acid isolation or biosensors.
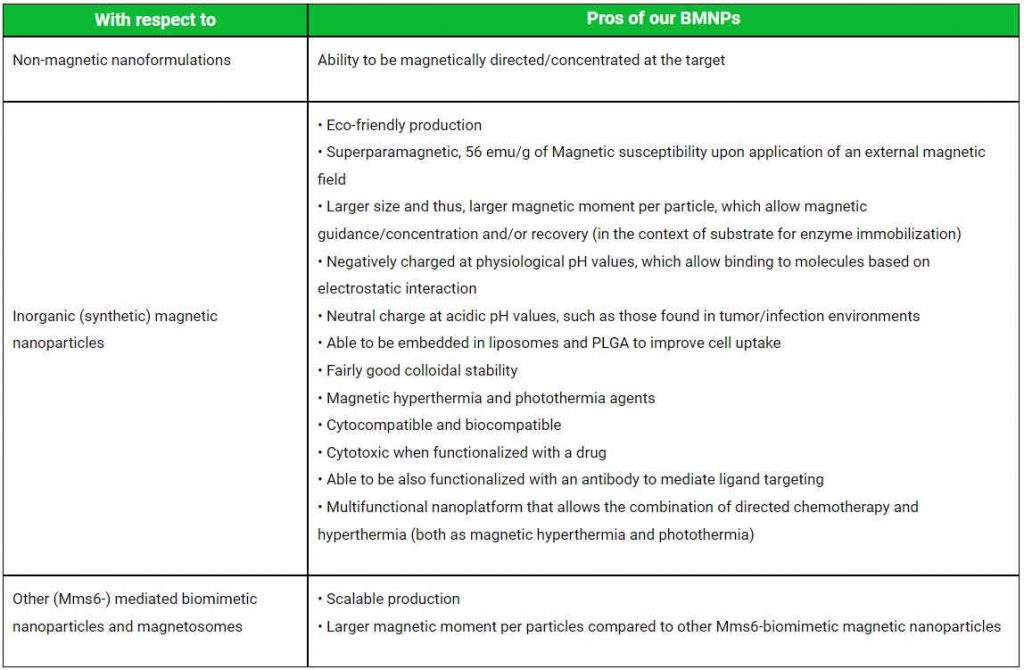
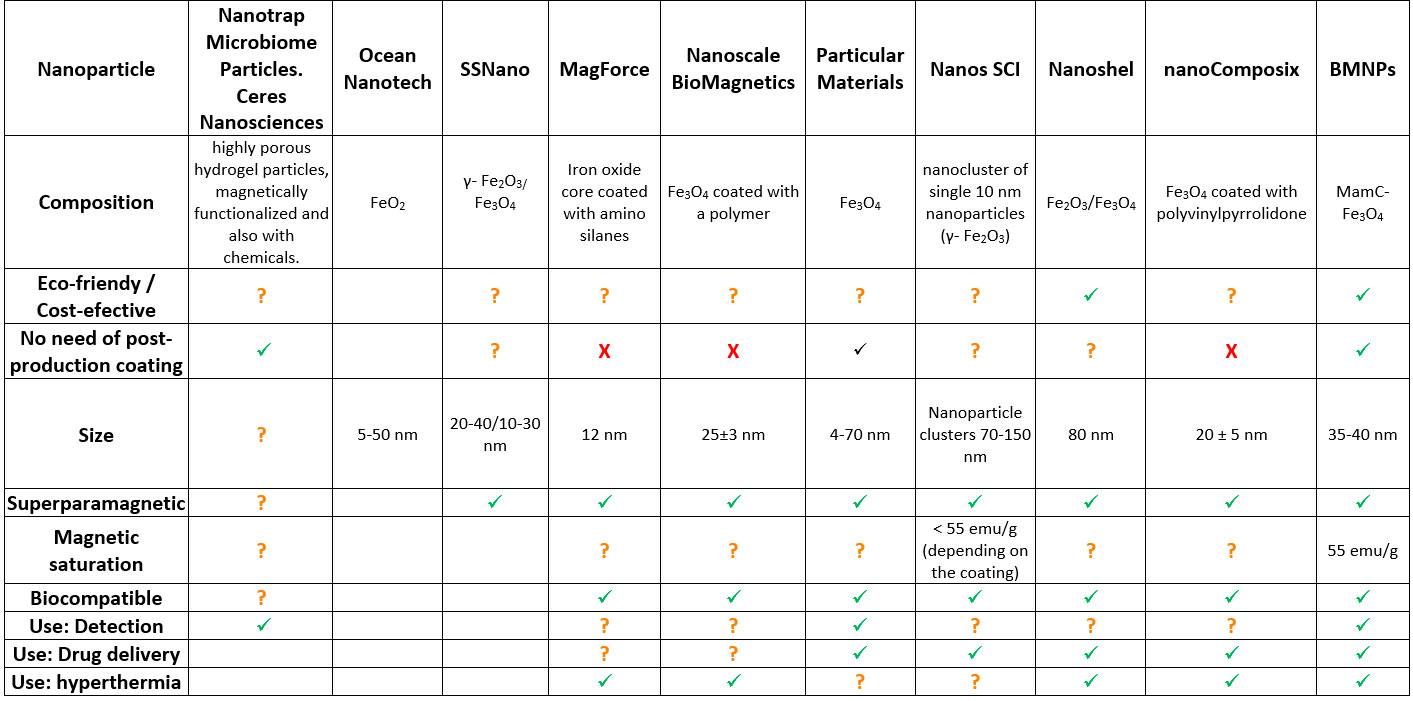
NOVELTY AND ADVANTAGES OF OUR NANOPARTICLE COMPARED TO THE EXISTING NANOFORMULATIONS
With respect to
Pros of our BMNPs
Non-magnetic nanoformulations
Ability to be magnetically directed/concentrated at the target
Inorganic (synthetic) magnetic nanoparticles
• Eco-friendly production
• Superparamagnetic, 56 emu/g of Magnetic susceptibility upon application of an external magnetic field
• Larger size and thus, larger magnetic moment per particle, which allow magnetic guidance/concentration and/or recovery (in the context of substrate for enzyme immobilization)
• Negatively charged at physiological pH values, which allow binding to molecules based on electrostatic interaction
• Neutral charge at acidic pH values, such as those found in tumor/infection environments
• Able to be embedded in liposomes and PLGA to improve cell uptake
• Fairly good colloidal stability
• Magnetic hyperthermia and photothermia agents
• Cytocompatible and biocompatible
• Cytotoxic when functionalized with a drug
• Able to be also functionalized with an antibody to mediate ligand targeting
• Multifunctional nanoplatform that allows the combination of directed chemotherapy and hyperthermia (both as magnetic hyperthermia and photothermia)
Other (Mms6-) mediated biomimetic nanoparticles and magnetosomes
• Scalable production
• Larger magnetic moment per particles compared to other Mms6-biomimetic magnetic nanoparticles
PROJECT FINANCE
Project PDC2021-121135-I00 financed by MCIN/AEI /10.13039/501100011033 and by the European Union Next Generation EU/ PRTR
2021 Proof of concept call (PDC2021). Resolution of the Presidency of the State Agency for Research in which is approved the call for granting funding to R+D+i projects for carrying out proofs of concepts tests within the framework of the R+D+i State Program devoted to Challenges in Society, from the State Program of Scientific and Technological Research and Innovation (2017-2020) (BOE nº 100, 27th April 2021).
Financial support for this investigation by Ministerio de Economía y Competitividad. CGL2004-03910, CGL2007-63859, CGL2010-18274, CGL2013-46612-P, CGL2016-76723, PID2019-109294RB-100, EC2019-005930-P, PDC2021-121135.100, Instituto de Salud Carlos III (PI20-01658), FEDER/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Spain (Grant P20_00346, P20_00208) and Consejería de Conocimiento, Investigación y Universidad, Junta de Andalucía, Spain (Grant A-FQM492-UGR20, A-BIO-376-UGR18, B-BIO-432-UGR20, B-CTS-216-UGR20) is gratefully acknowledged. Thanks also to grant TED2021-131855BI00 funded by MCIN/AEI /10.13039/501100011033